
|
Go To | Photo Products Technical Information |
LabLine Training Index |
Training Series
Part Four
Chemical Process Factors
The 3M LabLine Training Series is a regular feature of the LabLine which will provide a primer on many subjects related to
photofinishing technology, theory and application. The subject discussed in this and the next few issues will be Chemical Process
Quality Control. The study of the color negative film and the print from color negative paper processes, along with their monitoring and
control, will be presented in these issues. A general knowledge quiz on the subjects covered in each issue will also be included. The
information in this and following features will furnish the reader with a ready reference on the subject and supply a source of information
and materials to train new employees.
Introduction
The last issue of the LabLine Training Series discussed the color negative film and paper chemical processes.
It presented an introduction to the processes and discussed the role that each individual chemical step performed in the processing of
either film or paper. This issue of the series presents information on the principal processing factors of the actual chemical processes
used to produce prints from color negative films including the C-41 film process and the RA-4/EP-2 paper processes.
Processing Factors
Typically, a photo processing lab processes control strips in the processors which develop the
film and paper used to produce negatives and prints (a key part of process monitoring). This procedure is performed on a daily basis.
Information about the process is acquired through the densitometric measurement of the processed control strips by means of a
densitometer, and the comparison of this information to a standard (reference control strips). This information enables the lab operator
to evaluate the current level of the process including the level of each of the individual chemical steps. In other words, it provides the means
by which an operator can determine if the overall process is operating within acceptable limits.
s
Processing Factors directly affect the performance level or efficiency of the processing solutions in the chemical processes. The processing
equipment utilized by the lab must be set up according to exacting specifications for each of the processing factors. Processing factor specifications
are typically predetermined and published by the major manufacturers of film, paper and chemicals. Some specifications are built into the
processing equipment (for example, individual chemical processing times). Others, such as replenishment rates, are set and adjusted
by the lab operator.
The processes will be in control, producing good quality products, only when these
specifications are set properly in the processes. The primary goal in process monitoring is to produce an optimum quality product by
maintaining the process within these specifications. A change or shift in one of the processing factors will be detected when a control strip
is processed, measured and evaluated. That information, along with the operator's skill and experience, enables the lab operator to
determine what factor may need adjustment. The operator will then make those adjustments to the affected processing factor to bring
the process back in line or in control.
The principal processing factors are listed in the table below:
Time
The amount of time that the film or paper resides in an individual chemical solution will
affect the results of the final product. Developer times are the most critical. An increase or decrease in the developer time by a few seconds
can produce noticeable changes in the quality of the product. An increase in developer time will produce increased dye densities (high plots)
in the film or paper. Over developed negatives will produce high contrast prints and over developed paper will yield dark prints .
A decrease in developer time will produce decreased dye densities (low plots). This will yield low contrast prints from the under developed film or light prints from the under developed paper.
Most other solutions, such as bleaches, fixers, bleach-fixers, stabilizers etc., have a rather sizable
safety factor built into them. An increase in time generally has little or no effect. However, an excessive reduction in the time of these
solutions can result in them not completing their functions. For example, the bleach would not convert all the metallic silver to a non-metallic
form resulting in elevated Retained Silver readings (D-maxb-Yb) in the film process. This would produce washed out highlights and low
color saturation in the prints made from this film.
Excessively short film fixer times yield films which retain
silver halide compounds and produce prints which are low in contrast, color saturation and overall sharpness. Short stabilizer times in
the film process will produce films with low archival properties. This will allow the dyes in the film to deteriorate quickly over time. A short
bleach-fix time would result in muddy looking prints with little color saturation.
Times for each of the processing
solutions are generally built into the equipment and is determined by the speed of the drive motor, gear and sprocket sizes and rack lengths
in each of these machines. The operator of the equipment cannot usually control the time and it is therefore, not a factor that is normally
looked at if a control problem arises within the process.
Temperature
Temperatures are set
by the operator of the equipment to specifications provided by the manufacturer of the processing chemicals used in the equipment.
Temperature is generally not a factor looked at when a control problem arises provided the temperatures are set to the correct specifications
and the solution heaters and associated electronics are working properly. (NOTE: It is recommended that temperatures displayed by the
equipment are periodically checked for accuracy by actually measuring the temperature of the individual solutions with a good reference
thermometer. The temperature should be measured as near as possible to the center of the processing tank, and at least an inch below
the surface of the solution. If the actual measured temperature does not match the display, contact your equipment manufacturer for help
with adjusting the display. This is especially critical for developer solutions which have very narrow specification ranges, i.e. C-41
developer = 100±1/4° F.
However, the operator does control one system element that is related
to the performance of the tempering system; the chemical filtering system. In the vast majority of minilabs, the chemical filters need to
be maintained (cleaned or changed) on a regular basis to ensure proper processing temperatures. The chemical tempering in these type
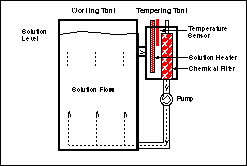
processors is done in small auxiliary tanks called "tempering tanks". These tanks are attached to the side of the main processing or
"working" tanks where the actual film or paper processing takes place. (See figure below.) A circulation pump constantly pulls solution from
the working tank into the tempering tank where it is tempered and filtered and, is then pumped back in to the working tank through a
turbulation system in the tank which provides agitation for the solution. The temperature sensors (which measure the actual temperature),
the solution heaters and the chemical filters are all located in these small tanks.

If the chemical filters are
dirty or clogged, the rate at which the solutions are exchanged between the tempering and working tanks is reduced. This results in working
tank temperatures that are actually lower than displayed temperatures. (Remember, the heater and sensor are in the small
tempering tank.) Low developer temperatures result in low plot densities and poor quality products.
Higher
than preset temperatures are not common. However, should a heater not turn off when required due to an equipment malfunction, the
temperature of the solutions would rise beyond specifications. If this occurred in a developer tank, the dye densities and plots would be
elevated. In secondary solutions such as bleach, bleach-fix, fixers or stabilizers, no effect would be noticed on plot densities. Elevated
temperatures will however, soften film and paper emulsions to a degree that they will scratch far more readily during processing than if
at proper temperatures.
Agitation
Agitation is simply the movement of chemicals within
the working tanks during the period when film or paper is processed. This is necessary in the process to insure that the film or
paper emulsions are constantly introduced to fresh chemicals in the working tanks. Without agitation, the film or paper would carry along
with it the first chemicals that it contacted when entering the solution. (See figure below.) In a very short amount of time, substantial
quantities of the active chemical compounds in the solution carried along by the film or paper, would be exhausted. This would have a
detrimental effect on the film or paper. If this were to occur in the developer tank, the rate of development would be severely reduced in
all or part of the film or paper. This would result in decreased dye densities, low plots and/or density streaks or spots on the film or prints.
Reduced agitation in secondary solutions also results in poor film or print quality due to streaking and mottling.
In most processors agitation is provided by the chemical circulation pumps. Agitation is produced as the
pumps circulate the chemicals between the tempering tanks and working tanks. The agitation of developer solutions is so critical that
many processors utilize an additional pump to supplement the agitation provided by the circulation pumps. It pulls developer out of the tank
and then pumps it directly back into the tank usually through a turbulator bar (a pipe with holes drilled in it) or other agitation device. Other
processors may bubble an inert gas such as nitrogen through the tanks to agitate them. Some solutions, such as C-41 bleach require air
agitation for proper results.
Specifications for agitation rates are published by the manufacturers of film and
paper photo products. Processor manufacturers install proper size pumps and agitation systems in their equipment which meet these
specification requirements.
Due to lack of operator control, agitation is generally not a factor that is looked
at when a control problem develops. However, in those machines utilizing a tempering tank, it is extremely important that the chemical filters
are well maintained. Failure to do so will result in restricted chemical flow between the tempering and working tanks, and therefore, reduced
agitation in the working tanks.
Chemical Strength
A developer solution may contain as many as
15 to 20 separate chemical compounds or constituents which work together to perform the development phase of
the C-41 or RA-4/EP-2 processes. Chemical Strength typically refers to the quantity of the chemical constituents within an
individual chemical solution. An example would be the quantity (i.e. grams per liter) of developing agent in a developer working tank solution.
Speci- fications for these quantities are established by the manufacturer of the chemicals during the research and development stages
of the product and are set with a specification and tolerance within which the chemical will yield optimum results for processing.
In order to produce high quality photofinishing products, developers and other solutions must be handled and
maintained properly by the lab operator so that the constituent levels within each solution remain within specifications. The typical lab does
not have the equipment required to actually measure the constituents in each of the chemical solutions. The lab operator can only
monitor the effects of solution constituent levels by processing control strips. Chemical strength is the factor that lab operators must
monitor closely to hold the process in control. Approximately 95% of processing problems can be attributed to chemical
strength changes.
There are several factors which control chemical strength levels in a photofinishing
environment. Replenishment Rate settings are generally considered the primary mechanism by which chemical strength levels
are controlled.
However, Chemical Mixing plays an equally critical, if not a greater role in the control of chemical strength levels.
Chemical Strength is also influenced by Contamination (the existence of constituents in a solution which are foreign to that solution).
Replenishment Rates
Replenishment rates refer to the rate at which fresh replenisher solutions are added to the working tank solutions to replace the
chemical compounds that are normally used up during the processing of film or paper. They also compensate for carry over, that is, as
film or paper travels from one solution to the next, it will carry some of the previous solution with it to the next solution thereby diluting that
solution. The replenisher solutions are generally stronger than the tank solutions. This minimizes the amount of replenisher that must be
added.
Film and paper chemical replenishment rate specifications are determined and published by the
manufacturer of the chemicals. Paper rates are usually stated in milliliters of replenisher per square foot of processed paper. For every
square foot of paper processed, a specific quantity of replenisher must be added back to the working tanks. Film rates are usually stated in
milliliters per linear foot or meter.
Published replenishment rates are based on normal equipment utilization.
Labs experiencing periods of low equipment utilization will find it necessary to adjust rates to compensate for the deterioration of chemicals
experienced during these periods. Most chemical manufacturers have recommendations for rates or procedures that should
be followed if your equipment is under utilized.
It is also extremely important that the lab
operator at start up top off working tanks with water to compensate for the evaporation of water from the solutions overnight. Failure to do
so will result in the equipment topping off the tanks with replenisher as film or paper is processed. This will produce an imbalance in the
chemical constituents in the working tank. Chemical byproducts are normally flushed out of the working tanks as fresh chemicals are
added during the replenishment cycle. This will not take place if tanks are not topped off.
A change in
replenishment rates is the most common method used to correct a process control problem. That is largely due to the fact that an incorrect
replenishment rate setting is the most common cause for a control problem. However, changing replenishment rates can mask other
problems such as low temperature, under agitation, improper chemical mixing procedures etc. It is always advisable to check all factors
before making a change in rates.
Chemical Mixing
Proper chemical mixing is one of the most
critical elements of good quality control in a photofinishing lab. A lab operator may be following all other procedures related to the
processing of film perfectly and still experience quality control nightmares if chemical mixing procedures are not followed correctly and
consistently. This one factor alone has the highest potential for increased costs in the lab. Improper mixing of chemicals will lead to high
paper waste, high chemical waste, increased labor costs and loss of business due to poor quality products produced in the lab.
Chemicals are packaged as concentrates which must be mixed with water. With the chemicals will be
packaged mixing instructions. The instructions will state the quantity and temperature of water that must be added to the concentrates to
form the proper size mix. They will state the order in which the individual parts must be added and often will have recommendations as
to the length of time each part should be mixed. These instructions must be followed as stated or quality control problems may result.
the most common mistake lab operators make is the addition of too much or too little water. The result is an
under concentrated or over concentrated replenisher solution. The process will react as if the replenishment rate was changed. Often, a lab
operator will adjust the rates only to have to perform this procedure again the next time chemicals are mixed. This results in roller
coaster plots and roller coaster quality.
It is also important that the correct temperature water is used when
mixing chemicals. Many concentrates will not dissolve properly in lower than recommended temperature water thereby resulting in under
concentrated replenisher solutions, which is also possible if the chemicals are not mixed long enough. Many chemicals, such as
developers, must not be mixed longer than recommended. Developing agents will oxidize if mixed too long. This will render them ineffectual
in the function for which they were formulated; developing exposed silver halide grains. Good quality and proper chemical mixing go hand in
hand, period.
Contamination
Contamination is the existence of a compound in a solution that is foreign to that solution. There are two types of contamination common
to the photographic processes. The first is a naturally occurring process whereby a film or paper traveling from one solution to the next,
will carry some of the previous solution to the following solution. Squeegees located between the solutions minimize this carry over and
replenishment rates take in consideration the resulting dilution from normal carry over. As the squeegees get worn, they will start to allow
more and more carry over. As an operator, there are two ways in which to eliminate the effect of this additional carry over. One is to increase
the replenishment rate of the chemical being diluted by the previous solution, or two, by replacing the worn squeegees or squeegee rollers.
By far the most cost effective means is to replace the squeegees. An increase in replenishment rates over a period of time becomes a
costly endeavor.
The second type of contamination results from a chemical that normally follows another in
the process is by some means deposited in a previous chemical step, i.e.. bleach or fixer or bleach/fix in a developer. This may cause a
serious problem with the control of the process. Iron is a common compound contained in bleaches. It only takes six parts per million
of iron deposited in a developer solution to have a noticeable photographic effect on the process. That's only a couple of drops of bleach
for most minilab processors. Slightly more than that will render the process out of control. It therefore is something to be avoided.
However, the contamination of developer solutions probably will occur in your lab at some time or another. It most commonly occurs when
racks are pulled for cleaning. Your equipment should have been supplied with splash guards by the manufacturer just for the purpose of
protecting solutions when pulling racks. If you don't know where they are, find them and use them. This alone will vastly reduce the
likelihood of this occurring.
If and when you do contaminate a solution, it is important that you follow a specific
procedure to minimize the loss. First, the contaminated tank should be drained and the chemical filter for this solution removed and
disposed of. Next, fill the tank with water and turn on the circulation system for this tank. This will flush any contaminated solution which
may be in the circulation lines or pumps. Then, drain and refill with a fresh tank mix and replace the chemical filter.
Next Issue:
Process Monitoring
Color Processing Quiz
Indicate whether the following statements are True or False.
- Photo processing labs run control strips in their chemical processes used to process film and paper on a daily basis.
T , F
- Control strips are evaluated by comparing them to the control strips which were processed the previous day on the processing equipment.
T , F
- Time is one of the processing factors that is easily adjustable in all types of processing equipment.
T , F
- If the developer time is too long, the densities on the control strips run in the process will probably be too high.
T , F
- The solution temperatures are preset in the equipment and cannot be changed.
T , F
- Dirty chemical filters can affect processor working tank temperatures.
T , F
- Agitation is required in a processor to maintain uniform solution temperatures.
T , F
- Lack of agitation may produce spots or streaks on film or paper.
T , F
- Approximately 95% of process control problems are the result of improperly mixed chemicals.
T , F
- Over utilized equipment requires lower than normal replenishment rates.
T , F
- It is extremely important to top off working tanks with water at equipment start up each morning.
T , F
- Chemical mixing is one of the most critical elements of good process control.
T , F
- Any temperature water may be used to mix most chemicals.
T , F
- Iron compounds will adversely affect the performance of a developer.
T , F
- If a solution is contaminated, the chemical filter should be cleaned.
T , F
Answers:
True: 1,4,6,8,11,12,14
False: 2,3,5,7,9,10,13,15
Go To | Training Guide 1 |
Training Guide 2 |
Training Guide 3 |
Training Guide 5 |
Training Guide 6 |

Copyright 1996 Imation. All rights reserved.













|





